According to science, fluoride is a nutrient which helps to prevent tooth decay and is added to the water supply in the interest of public health.
According to some people who aren't really good at understanding science, fluoride is a hazardous waste product (more toxic than lead!) which is added to our water by Big Dentistry to lower our children's IQs and destroy all our white blood cells. Also the Nazis used it! So there's that, too. (After visiting each of those sites, I hope to eventually study the relationship between "People Who Like Conspiracy Theories" and "People Who Don't Understand Graphic Design.")
And of course, according to General Ripper in Dr Strangelove, fluoride is a Commie plot to poison his precious bodily fluids:
Today's post was actually going to be about people who fear the chemicals in sunscreen and/or believe that suntanning has excellent health benefits, and the rest of us are just a bunch of "heliophobic" haters. I spent exactly ten minutes reading their blogs, realised that they were essentially setting their children up to suffer skin cancer, and retreated in disgust.
Instead, you get a post about water fluoridation, where the only side effect of ignorance is having your teeth fall out of your head. Let's break General Ripper's conspiracy theory down:
- "Do you realize that in addition to fluoridating water, why, there are studies underway to fluoridate salt, flour, fruit juices, soup, sugar, milk, ice cream? Ice cream, Mandrake? Children's ice cream!" Children's icecream, to the best of my knowledge, remains unfluoridated (except, of course, for any fluoridated water used in its manufacture). Deliberately added fluoride is found in municipal water supplies, and in toothpaste. It is worth noting that the protective effect of fluoride was discovered by observing the strong teeth of people who lived in areas where the water contained naturally high levels of fluoride - ten times higher than that found in fluoridated municipal water supplies. In other words, fluoridated water also occurs as an entirely natural substance.
- "...You know when fluoridation began?...1946. 1946, Mandrake. How does that coincide with your post-war Commie conspiracy, huh? It's incredibly obvious, isn't it?" Fluoridation was actually introduced in 1945, although in the Australian state of Queensland it wasn't introduced until 2008. This clearly goes some way to explaining the heightened intelligence, and indeed life essence, of Queenslanders.
- "A foreign substance is introduced into our precious bodily fluids without the knowledge of the individual, and certainly without any choice. That's the way your hard-core Commie works." The fluoridation of water is not a secret. Scientists, dentists and governments are justifiably proud of the public health benefits of fluoridation. However, if one does not wish to partake of government-fluoridated water, then I suppose you could just dig a well in your backyard? Unless your local groundwater has fluoride in it, in which case I guess you just die of dehydration with your precious bodily fluids uncompromised.
- "I first became aware of it, Mandrake, during the physical act of love... Yes, a profound sense of fatigue, a feeling of emptiness followed. Luckily I — I was able to interpret these feelings correctly. Loss of essence. I can assure you it has not recurred, Mandrake. Women, er, women sense my power, and they seek the life essence. I do not avoid women, Mandrake...but I do deny them my essence." Portland is the largest US city to remain unfluoridated (until 2014). It's also the US city with the highest rate of depression. Some might say it's caused by the 222 cloudy days per year, or the insufferable hipsters. Either way, it appears that even without fluoride, their life essence is faltering.
Seriously, though: fluoride is added to water supplies for the same reason iodine is added to salt, or Vitamin D is added to milk, or folic acid is added to flour: it's a cheap, effective, equitable way to provide an important health benefit to an entire population. Fluoridation generally costs less than $1 per person per year, and each dollar spent saves $38 in further dental costs. Fluoridation is credited with a 40-70% decrease in tooth decay in children, and a 40-60% decrease in tooth loss for adults. Even with fluoridation, 51 million school days per year are lost in the US due to dental illnesses, a number which would be much higher without fluoridation.
Fluoridation is considered one of the ten greatest public health achievements of the last century, along with vaccination and the recognition of cigarettes as a health hazard.
Fluoridation is considered one of the ten greatest public health achievements of the last century, along with vaccination and the recognition of cigarettes as a health hazard.
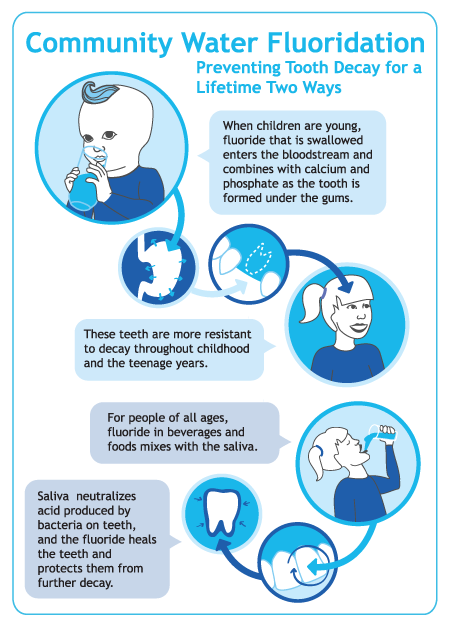 |
| I enjoy this graphic both for its pertinent information, and for the fact that the baby looks like a unicorn. |
I suspect there is very little overlap between people who have hysterics over their precious bodily fluids, and people and children who would not otherwise be able to afford dental care. Those who claim that they should be able to choose to take fluorine in tablets (as is done in communities reliant on rainwater tanks) are advocating for an alternative which is wasteful - in packaging, manufacture, and the time of doctors and pharmacists - as well as less accessible to people without the privilege of money or health education.
If you have the money to pay for a dentist, then you have the money to buy one of the many filters available to remove fluoride from your water. So go do that, and...

If you have the money to pay for a dentist, then you have the money to buy one of the many filters available to remove fluoride from your water. So go do that, and...






















